In July 2015, the FDA approved alirocumab (Praluent), the first cholesterol-lowering treatment in a new class of drugs known as proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors. The following month, the FDA approved the second PCSK9 inhibitor, evolocumab (Repatha).
This article highlights several key therapeutics areas for Praluent and Repatha that every pharmacist should know.
Indications
Praluent is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of low-density lipoprotein cholesterol (LDL-C).
Repatha is indicated for those same indications, plus as an adjunct to diet and other LDL-lowering therapies for the treatment of patients with homozygous familial hypercholesterolemia (HoFH) who require additional lowering of LDL-C.
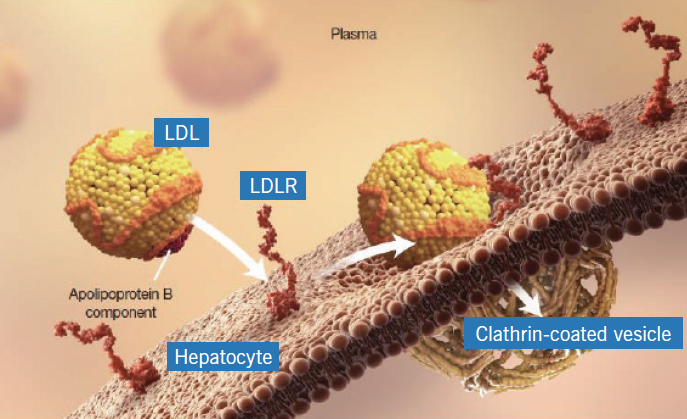
Mechanism of Action
Alirocumab and evolocumab are both human monoclonal antibodies that bind to PCSK9. Under normal conditions, PCSK9 binds to the LDL receptors (LDLR) on the surface of hepatocytes to promote LDLR degradation within the liver. Because LDLR is the primary receptor that clears circulating LDL, the decrease in LDLR levels by PCSK9 results in higher blood levels of LDL-C.
By inhibiting the binding of PCSK9 to LDLR, these medications increase the number of LDLRs available to clear LDL, thereby lowering LDL-C levels in the body.
Formulation
Praluent is supplied in single-dose, prefilled pens and glass syringes. Each prefilled pen or syringe is designed to deliver 1 mL of 75 mg/mL or 150 mg/mL solution. Praluent is available in cartons containing 1 or 2 prefilled pens and 1 or 2 prefilled syringes.
Repatha is supplied in a single-use, prefilled syringe or SureClick auto-injector. Each prefilled syringe or auto-injector is designed to deliver 1 mL of 140 mg/mL solution. Repatha is available in packs of 1, 2, and 3.
Dosing
The recommended starting dose for Praluent is 75 mg administered subcutaneously once every 2 weeks. If the LDL-C response is inadequate, the dosage may be increased to the maximum dosage of 150 mg administered every 2 weeks.
The recommended starting dose for Repatha varies by indication. For primary hyperlipidemia with established clinical ASCVD or HeFH, it should be dosed at 140 mg every 2 weeks or 420 mg once monthly in the abdomen, thigh, or upper arm. For HoFH, the recommended dose is 420 mg once monthly.

Efficacy
Praluent’s approval was based on 5 double-blind, placebo-controlled trials that enrolled a total of 3499 patients (36% with HeFH, 54% without FH who had clinical ASCVD). All patients received a maximally tolerated dose of a statin with or without other lipid-modifying therapies.
In all trials, Praluent resulted in statistically significant reductions in LDL-C levels, ranging from a 39% to 59% reduction.
Repatha’s approval was based on 4 double-blind, placebo-controlled trials that enrolled a total of 813 patients. Two of studies were conducted in patients with clinical ASCVD, 1 in patients with HeFH, and 1 in patients with HoFH. All patients received a maximally tolerated dose of a statin, with or without other lipid-modifying therapies.
In all trials, Repatha resulted in statistically significant reductions in LDL-C levels, ranging from a 31% to 61% reduction.
Safety
The most common adverse reactions related to Praluent and Repatha are nasopharnygitis, injection site reactions, influenza, urinary tract infection, diarrhea, bronchitis, myalgia, muscle spasms, and sinusitis. Additional potential safety concerns with PCSK9 inhibitors include neurocognitive issues, new-onset diabetes mellitus, neural tube defects, and immunogenicity.
Some medical experts have expressed concern about PCSK9 inhibitors lowering LDL-C levels too far. This concern was also raised while these medications were reviewed during an FDA advisory committee meeting.
Storage
Both Praluent and Repatha should be stored in a refrigerator in their original carton to protect from light. Alternatively, Repatha can be kept at room temperature in the original carton; however, it’s not recommended to keep it longer than 30 days before discarding.
Praluent should be allowed to warm to room temperature for 30 to 40 minutes prior to use. Unlike Repatha, however, the product should be discarded if left at room temperature for 24 hours or longer.
Guideline Recommendations
Current US cholesterol guidelines have not yet been updated to determine PCSK9 inhibitors’ place in therapy. However, the 2016 American Diabetes Association guidelines say they may be considered as adjunctive therapy for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL-C or are intolerant to high-intensity statin therapy.
Further Study
Praluent is currently being studied in the ODYSSEY Outcomes trial, which is designed to determine whether the addition of Praluent to intensive statin therapy reduces major adverse cardiac events among patients who had previously experienced an acute coronary syndrome. The 18,000-patient trial is expected to be completed in 2017.
PDF link National Lipid Association
Repatha is currently being studied in the FOURIER outcomes trial, which is designed to evaluate whether Repatha in combination with statin therapy, compared with placebo plus statin therapy, reduces the risk of cardiovascular events in approximately 27,500 patients with high cholesterol and clinically evident CVD. Results from the study are anticipated in the second half of 2016.
Conclusion
Although Praluent and Repatha have been shown to be extremely effective in reducing LDL-C levels, little evidence is currently available on improvements in clinical outcomes and long-term safety data. The results of further studies that address those concerns will be published in the next few years.
[slideshow_deploy id=’159′]

