by Vita | سبتمبر 24, 2016 | News
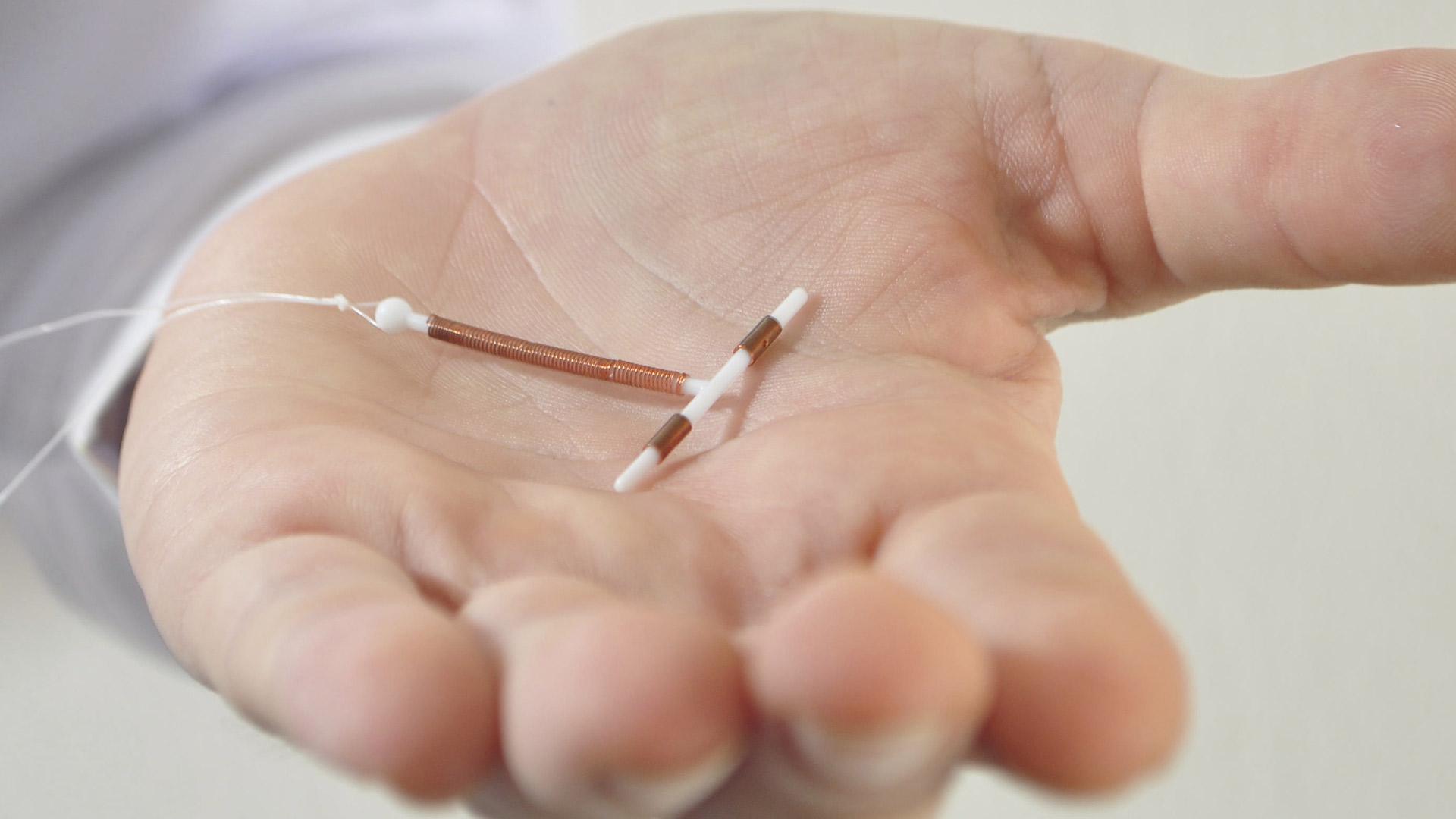
Bayer announced today that the U.S. Food and Drug Administration (FDA) approved Kyleena (levonorgestrel-releasing intrauterine system) 19.5 mg, a progestin-containing intrauterine system (IUS), for the prevention of pregnancy for up to five years.1Kyleena will be...


